T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is a rare and aggressive variant of large B-cell lymphoma. A substantial subset of THRLBCL patients (pts) relapse after frontline anthracycline-based therapy. There is a scarcity of outcome data on therapy options for relapsed/refractory (r/r)THRLBCL, particularly CD19 CAR-T therapy.A recent genetic and spatial analysis provided critical insights into the complex interactions at the tumor-immune interface in THRLBCL. Understanding the mechanisms by which tumor cells evade the immune system is crucial in designing treatment strategies. This retrospective study aims to analyze clinicopathologic data in pts with r/r THRLBCL with the goal of identifying patterns that may influence response rates and long-term survival.
METHODS: Pts who were evaluated and treated at MD Anderson Cancer Center (MDACC) with THRLBCL from 1/2005-5/2023 were included in the analysis. We reviewed demographic, clinical and pathological characteristics, treatments, and outcomes through an approved IRB protocol. We excluded cases with ambiguous diagnosis (such as DLBCL with increased infiltrate of T-cells and/or histiocytes), missing treatment, and/or no follow up information. Progression-free survival (PFS) and overall survival (OS) were calculated from start of treatment until progression or death using Kaplan-Meier. Log-rank test was used to evaluate the difference between pt groups. Statistical software SAS 9.4 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for all analyses.
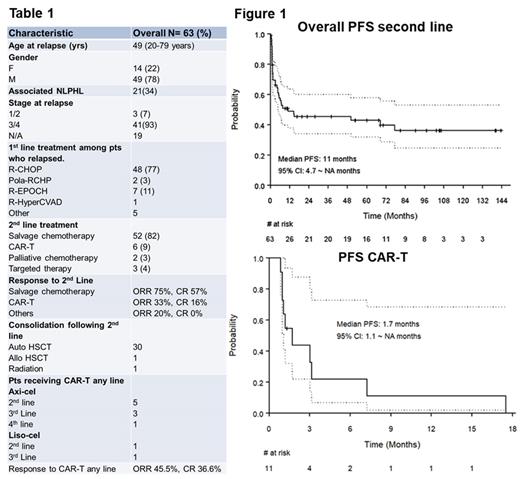
RESULTS: A total of 212 pts were reviewed, and we excluded 86 pts who did not meet selection criteria. Out of 126 pts, 63 with biopsy-confirmed relapse after 1st line chemoimmunotherapy were included in the analysis (Table-1). The median age was 49 (20-79 years); and 49 pts (78%) were males. Twenty-one (34%) pts had documented NLPHL at the time of or predating the THRLBCL diagnosis. At relapse, 93 % had advance stage disease (stage 3 and 4). Splenic involvement was documented in 57% and 80% of pts had one or more sites of extra nodal disease. Most pts who relapsed received front line RCHOP (77%) or R-EPOCH (11%). Twenty-six pts (41%) had primary refractory disease, and 24 pts (38%) relapsed within 12 months. The median time to progression from 1st line treatment was 7.4 months(2.2~145.9 months).
The most common 2 nd line therapy was salvage chemotherapy (82%) such as R-ICE (n=41), R-DHAP(n=4), R-GemOx (n=3). Overall response rate (ORR) to 2 nd line salvage chemotherapy was 75% with CR of 57%. For pts who received 2nd line salvage chemotherapy with the intent to undergo HSCT, the PFS at 2 years was 53% (95% CI: 0.4-0.68, p< 0.0001) and OS was 64% (95% CI: 0.5- 0.8, p< 0.372). Among pts who received consolidation with HSCT, (n=30, 57%), 5 pts relapsed at the time of last follow up.With a median follow-up time of 60.1 months (95% CI: 33.6~90.3 months), the median PFS from all 2nd line treatments was 11 months (95% CI: 4.7 ~ NA ) and for pts who received salvage chemotherapy with HSCT intent was 50.26 (95% CI: 7 ~ NA months).
The median OS from 2nd line for all pts was 77.3 months (95% CI: 22.2 ~ NA months) and for pts who received salvage chemotherapy with HSCT intent OS was not reached (95% CI: 32 ~ NA months). Among 11 pts who received 3 rd line salvage chemotherapy, the median PFS was 2 months, and only one pt received HSCT. All pts eventually had progressive disease.
Eleven pts received CD19 CAR-T (Axi-cel-9, Liso-cel-2),(table-1) 6 pts received as 2 nd line, 4 received as 3rd line and 1 pt received as 4 th line. Best ORR to CAR-T at any lines was 45% with CR of 36.4%. At a median follow-up time of 12.3 months (95% CI: 2.4 ~ NA months), all but one pt had disease progression. Median PFS was 1.7 months (95% CI: 1.1 ~ NA months). 6-month PFS was 21% (Figure 1). One pt who had response experienced prolonged remission of 17 months before relapsing.
CONCLUSION: Our study highlights the unique biology and poorer prognosis of TCHRBCL compared to other DLBCL subtypes. Despite the success of CD19 CAR-T therapy in DLBCL, TCHRBCL pts have shown limited response to these therapies, demonstrating the challenges of achieving long-term remission in this group. Our study shows the high unmet need for improved therapies that can target the unique biology in TCHRBCL. We plan to report a comparative spatial analysis evaluating the tumor-immune interface that can contribute to resistance to Immune effector cell therapies at the meeting.
Disclosures
Jain:AstraZeneca: Consultancy, Honoraria. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Pinnix:Merck: Research Funding. Steiner:Seattle Genetics: Research Funding; Bristol Myers Squibb: Research Funding; Rafael Pharmaceuticals: Research Funding; GSK: Research Funding. Lee:Aptitude Health: Honoraria; Bristol-Myers Squibb: Research Funding; Cancer Experts: Honoraria; Celgene: Research Funding; Century Therapeutics: Consultancy; Curio Sciences: Honoraria; Deloitte: Honoraria; Guidepoint: Honoraria; Janssen: Honoraria; Korean Society of Cardiology: Honoraria; Olson Research: Honoraria; Oncternal Therapeutics: Research Funding; Pharmacyclics: Research Funding; Seagen Inc.: Research Funding; Takeda: Research Funding. Strati:Roche Genentech: Consultancy; Kite Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Hutchinson MedoPharma: Consultancy; Astrazeneca Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; ALX Oncology: Research Funding. Nieto:Affimed: Research Funding; Astra Zeneca: Research Funding; Secura Bio: Research Funding. Green:Abbvie: Research Funding; Kite/Gilead: Research Funding; Sanofi: Research Funding; Allogene: Research Funding; KDAc Therapeutics: Current equity holder in private company; Daiichi Sankyo: Honoraria; BMS: Consultancy; Abbvie: Honoraria. Neelapu:Kite, A Gilead Company: Consultancy, Other: Advisory Board Member, Research Funding; Merck: Consultancy, Other: Advisory Board Member; Sellas Life Sciences: Consultancy, Other: Advisory board member; Athenex: Consultancy, Other: Advisory board member; Allogene: Consultancy, Other: Advisory board member, Research Funding; Incyte: Consultancy, Other: Advisory board member; Adicet Bio: Consultancy, Other: Advisory board member, Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board Member, Research Funding; Bluebird Bio: Consultancy, Other: Advisory board member; Fosun Kite: Consultancy, Other: Advisory board member; Sana Biotechnology: Consultancy, Other: Advisory board member, Research Funding; Caribou: Consultancy, Other: Advisory board member; Astellas Pharma: Consultancy, Other: Advisory board member; Morphosys: Consultancy, Other: Advisory board member; Janssen: Consultancy, Other: Advisory board member; Chimagen: Consultancy, Other: Advisory board member; Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; Orna Therapeutics: Consultancy, Other: Advisory board member; Takeda: Consultancy, Other: Advisory board member; Synthekine: Consultancy, Other: Advisory board member; Carsgen: Consultancy; Precision Biosciences: Research Funding; Longbow Immunotherapy: Current holder of stock options in a privately-held company; N/A: Patents & Royalties: Related to cell therapy and the safety switch described (intellectual property). Vega:Geron: Research Funding; Allogene: Research Funding. Flowers:Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Genentech Roche: Consultancy, Research Funding; Genmab: Consultancy; Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Pharmacyclics Jansen: Consultancy; SeaGen: Consultancy; Spectrum: Consultancy; 4D: Research Funding; Acerta: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Iovance: Research Funding; Jannsen Pharmaceuticals: Research Funding; Kite: Research Funding; Morphosys: Research Funding; Nektar: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Westin:Kite/Gilead: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Abbvie: Consultancy; SeaGen: Consultancy; Nurix: Consultancy; MonteRosa: Consultancy; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Calithera: Research Funding; Kymera: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal